Hi, I’ve added some more info on the Orders page and under the Transition metals heading. Go check them out!
Epiphany Chemistry
05 Jan 2016 Leave a comment
We’ve all heard the story of the three wise men bringing Jesus gifts of gold, frankincense and myrrh. But for many of us those last two words don’t mean an awful lot. Of course, gold is more obvious, it’s a chemically unreactive, soft, precious metal used most often in jewellery. Its chemical symbol is Au after its latin name, aurum.
Frankincense
Extraction:
Frankincense comes from the sap of the Boswellia Sacra tree in the Arabian peninsula. The bark is cut so that a milky white sap comes out, this first sap is then discarded. The sap collected after further cutting is then dried into solid yellow droplets.
Uses:
Frankincense has been burned as incense since the ancient Egyptians, Romans and Greeks, the smoke makes a thin black line that rises straight upwards, symbolising the prayers reaching up to the gods. It is also a very effective skin care oil.
Composition:
Frankincense is actually a mixture of hundreds of different compounds and the composition varies depending on climate and geography because these factors affect the biochemical processes in the plants. The most common molecules in frankincense are boswellic acids which are pentacyclic triterpene molecules. So basically a big, complicated organic compound. Frankincense’s aroma comes from terpenes such as limonene and pinene.
Myrrh
Extraction:
Myrrh is extracted in a similar way to Frankincense. Myrrh is the resin of a number of small, thorny tree species of the genus Commiphora, it is a natural gum and is most commonly harvested from the Commiphora Myrrha which is native to Yemen, Somalia, Eritrea and eastern Ethiopia.
Uses:
Myrrh was associated with both healing and embalming. It was used in perfumes, medicines, and as a way to preserve corpses- particularly by the ancient Egyptians who used Myrrh and Natron for the embalming of mummies. To this day, myrrh is used as an antiseptic in mouthwashes and toothpastes, in liniments and healing salves for minor skin ailments and as an analgesic for toothache.
Composition:
Myrrh is also made up of many different compounds. According to http://www.herballegacy.com/Knottnerus_Chemical.html, myrrh is ‘about 9 to 17% volatile oil, 20 to 40% alcohol-soluble resin, and approximately 30 to 60% water-soluble gum (ABC).’ With furanosesquiterpenes such as furanoeudesma-1,3-diene and other molecules like lindestrene and dihydropyrocurzerenone providing myrrh’s smell.
What I’m up to
14 Dec 2015 Leave a comment
At the moment most of the work I’m doing on this site is on the pages under organic chemistry rather than blog posts. So go exploring 🙂
Response to Telegraph Article – ‘My daughter shouldn’t have to study science’
11 Nov 2015 Leave a comment
‘My daughter shouldn’t have to study science’, says Cristina Odone
Too many girls are pressurised into taking ‘STEM’ subjects just to appease feminists, argues Cristina Odone
This article intrigued me because I must have changed my mind at least five times whilst reading it. Odone argues that strong, feminist views encouraging girls to take STEM subjects have gone too far, to the point at which it ‘By rejecting isosceles triangles and Bunsen burners, she was betraying the suffragettes and Marie Curie.’ Not everybody has an aptitude for science and maths, and of course not everybody enjoys these subjects. So are we pushing girls into typically ‘male’ subjects regardless of whether it is really the best thing for them?
On the other hand, isn’t it worrying that ‘women make up only 12.8 per cent of the STEM workforce.’? Ideally, we should be aiming for equality and that may be mean encouraging girls to take STEM subjects, but it also means not forcing them into a subject in a way that we wouldn’t a boy.
Phenols
30 Oct 2015 Leave a comment
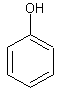
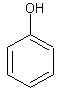
Phenols are a type of organic compound in which there is a benzene ring with a hydroxyl (-OH) group is directly attached. If the hydroxyl group is attached indirectly to the benzene group, the molecule is not a phenol.
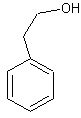
The image on the left shows phenol, the simplest form a phenol can take. So there is a group of molecules called phenols and the simplest of these is called phenol, just to cause confusion. There is simply one benzene ring with a hydroxyl group attached directly to one of the carbons in the ring. If there were other groups attached to the other carbons in the ring, the molecule would still be a phenol. For example, 2,4-dimethylphenol would be the molecule shown on the left but with a CH3 group on the second and fourth carbons in the ring. This molecule would still be classed as a phenol because the hydroxyl groups is still attached directly to the benzene ring. The molecule on the left however is not a phenol. In C6H5CH2CH2OH, or 2-pheylethanol, the hydroxyl group is attached to the ethyl group which is attached to the benzene ring, so it is not attached directly to the benzene ring.
Let’s look at the properties of phenol. As the simplest of the phenols, we use this molecule to describe the general behaviour of all phenols. Phenol is a solid at room temperature and pressure. Because the hydroxyl group can form hydrogen bonds with water moelcules, phenol is slightly soluble in water, however the benzene ring means that it is less soluble in water than alcohols.
Reactions of phenol
Reaction with sodium hydroxide:
When dissolved in water, phenol forms a weak acid by the dissociation of the H+ and C6H5O–. Phenol is neuralised by aqueous sodium hydroxide to form the salt sodium phenoxide and water.
C6H5OH + NaOH → C6H5O–Na+ + H2O
Reaction with sodium:
When a reactive metal such as sodium is added to phenol, the metal effervesces producing hydrogen gas. The organic product, sodium phenoxide, is a salt.
2C6H5OH + 2Na → 2C6H5O–Na+ + H2
Notice that both of these reaction form sodium phenoxide, C6H5O–Na+.
Reaction with bromine:
This reaction, just like that of benzene and bromine is electrophilic substitution, but unlike that reaction, the reaction of phenol and bromine takes place at room temperature and does not need a halogen carrier catalyst. In this reaction, the orange bromine colour disappears and a white precipitate is formed.
The reason for this difference between benzene and phenol is the lone pair in the hydroxyl group. The lone pair is drawn into the cloud of delocalised electrons which increases the electron density in the ring. This means that the reaction happens much faster as the increased electron density polarises bromine molecules quicker.
Reactions of benzene
28 Sep 2015 Leave a comment
I’ve just realised that although I’ve covered the discovery of the structure of benzene, I haven’t blogged about its reactions!
Benzene reacts differently to the typical double-bond functional groups. This is because the cloud of delocalised electrons form a very stable structure which is difficult to challenge: benzene isn’t going to react in a way that makes it less stable!
In normal conditions, benzene does not:
- decolourise bromine water
- react with strong acids such as HCl
- react with the halogen chlorine, bromine or iodine
All of the above reactions would be expected from alkenes, but benzene is different. If benzene took part in an additions reaction, it would need to bond to the atom or groups of atoms being added, ultimately disrupting the delocalised structure, however in substitution reactions the cloud of delocalised electrons is maintained.
In benzene, there are two areas of high electron density; above and below the plane of the carbon atoms. These areas attract electrophiles, meaning that benzene takes part in many electrophilic substitution reactions.
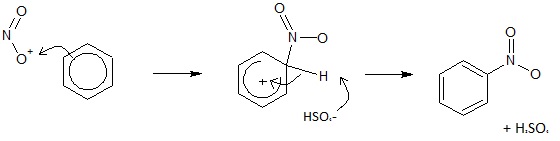
The nitration of benzene is an electrophilic substitution reaction that is very common. In this reaction, benzene becomes nitrobenzene, with a hydrogen atom being replaced by a nitro (-NO3) group. Benzene is reacted with a nitrating mixture made up of concentrated nitric acid and concentrated sulfuric acid at a temperature of 50o. The sulfuric acid is a catalyst in this reaction.
Rate of Reaction
02 Sep 2015 Leave a comment
In AS chemistry, the term ‘rate of reaction’ refers to some vague concept of how fast a reaction is happening. For AS, we use this term to discuss the possible effects different conditions will have on the ‘rate of reaction’. But what actually is the ‘rate of reaction’? How can we measure it? Thankfully, A2 chemistry seems to have some answers.
The ‘rate of reaction’ does essentially mean how fast the reaction is, but more specifically, it is to do with the rate at which reactants are used up and products are made.
rate = change in concentration of reactant or product/time for the change to take place
The unit for the rate of a reaction is usually mol dm-3 s-1, however it could be any unit which represents concentration/time, to complicate matters though, it doesn’t have to be concentration! It can be a different physical quantity that is proportional to concentration, for example, volume; cm3 min-1.
The rate of reaction will change throughout the course of the reaction, even if all the conditions are kept the same. This is because the concentration of reactants will decrease as the reaction proceeds, so the number of successful collisions per second will also decrease.
The rate of a reaction can be displayed in a concentration-time graph. The concentration of either the reactants or products is measured at set intervals during the experiment. At any instant of time, the rate is equal to the slope of the curve. The slope of the curve can be measured by drawing a tangent to the curve at this time and working out the gradient of this tangent: change in y/change in x.
Nisyros Volcano
01 Sep 2015 Leave a comment
 I have just returned from a holiday in the Greek Island of Kos. Near the beginning of the week, we went on an excursion to the volcanic island of Nisyros. Nisyros is still active and has beautifully colourful craters with some very active fumaroles. The stench of sulfur was very overpowering at first but we managed to deal with it long enough to walk down into the crater and get some great snaps.
I have just returned from a holiday in the Greek Island of Kos. Near the beginning of the week, we went on an excursion to the volcanic island of Nisyros. Nisyros is still active and has beautifully colourful craters with some very active fumaroles. The stench of sulfur was very overpowering at first but we managed to deal with it long enough to walk down into the crater and get some great snaps.
The Aegean Plate which Greece is part of is inbetween the Turkish, Euras ian and African Plates. These three plates are all moving more or less into the Aegean Plate, so it is not too surprising that there is a large amount of volcanic activity in this area. Nisyros is art of the Hellenic Arc; an arc containing several active volcanoes such as Methana, Santorini, and the Bodrum Peninsula.
ian and African Plates. These three plates are all moving more or less into the Aegean Plate, so it is not too surprising that there is a large amount of volcanic activity in this area. Nisyros is art of the Hellenic Arc; an arc containing several active volcanoes such as Methana, Santorini, and the Bodrum Peninsula.
Fumaroles
‘Fumaroles are vents from which volcanic gas escapes into the atmosphere.’ (http://www.volcanodiscovery.com/what-is-a-fumarole.html) They are present on active volcanoes during periods of relative quiet between eruptions. The temperatures of the fumaroles at Nisyros have been changing; from 2000 to 2004, the temperatures rose from 98oC to 103oC. A magma chamber below Nisyros is only 3-4 km deep and it is sti ll rising. Fumaroles can be very dangerous, not only because of the high temperatures but also because of the poisonous gasses which they release. Normally, they are mostly water vapour from groundwater which is heated by magma, the magma itself releases gasses such as carbon dioxide, sulfur dioxide, and hydrogen sulfide. There are also many hot springs around the island’s coast which have been used for bathing and healing.
ll rising. Fumaroles can be very dangerous, not only because of the high temperatures but also because of the poisonous gasses which they release. Normally, they are mostly water vapour from groundwater which is heated by magma, the magma itself releases gasses such as carbon dioxide, sulfur dioxide, and hydrogen sulfide. There are also many hot springs around the island’s coast which have been used for bathing and healing.
Review of article from ‘Chemistry World’
30 Jun 2015 Leave a comment
‘Airborne pesticides need surveillance’
Summary:
Some scientists, especially Susan Kegly, a chemist at the Pesticide Action Network North America, are becoming increasingly worried about the effects of airborne pesticides. Airborne pesticides are not just found in the air immediately around crops but also in the wider surrounding area. In California, this is a big concern because it has more crops than any other state and 30% of the total US pesticide use is in California. In communities where there is a high concentration of pesticides in the air, there is concern about the impacts of this on people’s health, and it has been suggested that pesticides may have some link to cancer.
There are many cases like this in science where people are warned against something because of the possible health effects. We are told not to live near telephone poles, not to work the night shift, not to use birth control pills etc because it might give us cancer. But is there actually any truth in this, or is it just scaremongering? However, even if there is the slightest chance that something does pose a health risk, should we not do whatever we can to minimise this risk? As Kegly says, ‘If there is another way to do it, why are we still using spray pesticides?’ In the case of pesticides, the evidence to suggest that they are associated with certain health issues is building up, so why is the EPA not doing anything?